
Jacqueline Collins

Audio By Carbonatix
The state’s hemp industry, which produces the majority of cannabidiol (CBD) products in Colorado, are worried about the consequences of a bill the General Assembly has approved that opens the door for CBD medicine approved by the United States Food and Drug Administration.
Colorado lawmakers wanted to be ahead of the curve with HB 1187, introduced by State Representative Janet Buckner, to allow a pharmaceutical drug made from CBD that is expected to receive FDA approval within the next couple of months to be sold in Colorado. That drug, Epidiolex, is made by an American subsidiary of British GW Pharmaceuticals for treating epilepsy.
And not all of Colorado’s hemp industry is on board with the special treatment.
“Our stakeholders definitely have issues with the GW bill,” says Veronica Carpio, founder of hemp industry trade group Grow Hemp Colorado. “We know we can’t stop that and other cannabis-related medicine. We just wanted one year to hold off to see the consequences coming down the federal pipeline. We weren’t in the hurry that GW Pharma was.”
Anticipating the FDA’s blessing of the drug, GW Pharmaceuticals, which didn’t respond to requests for comment, has been lobbying for the bill to pass during this legislation session. If the measure hadn’t passed or is vetoed by Governor John Hickenlooper, who still needs to sign the bill, GW Pharmaceuticals will have to wait until the 2019 legislative session or for a federal rescheduling of cannabis to be able to dispense the drug. In the meantime, Carpio says her group’s members are prepared to provide free CBD oil to medical patients who would have been prescribed Epidiolex.
Because cannabis is still a Schedule I substance in the eyes of the federal government, physicians can’t prescribe any medicine derived from the plant even if that medicine has FDA approval, and pharmacists can’t dispense it, either. The bill essentially intends to give Epidiolex a pass similar to Marinol, a pharmaceutical that uses synthetic THC and received a carve-out in federal law to avoid breaking the Controlled Substances Act. This is an entirely new piece of legislation for a plant-based medication, however, and Carpio worries it could hurt Colorado’s burgeoning hemp and CBD industries, both of which are currently unregulated by the FDA.
Carpio feels the bill could “open Pandora’s Box” by prescribing something that is currently outlawed by the Drug Enforcement Administration, and is still worried there isn’t enough protection in the bill from unintended consequences that could lead federal agencies to crack down on non-FDA-approved CBD and hemp products that are currently sold in Colorado.
Not all of the hemp industry has opposed the bill. Hoban Law Group, which represents businesses in the hemp and marijuana industries, along with the NoCo Hemp Expo and marijuana lobbyist Cindy Sovine, have supported the bill on the expectation that Colorado’s hemp industry would be protected by the bill’s guardrails.
After attending a stakeholder meeting called by Representative Jonathan Singer in March, Carpio thought that HB 1187 would be killed and a new, more simplified bill would be introduced.
The meeting included Carpio, representatives Buckner and Singer, and a GW Pharmaceuticals representative, among others. Instead of mentioning “marijuana” or “cannabidiol” specifically, the potential alternative bill would have simply allowed those with prescription-writing authority to prescribe FDA-approved drugs and pharmacists to dispense them. Everyone was on board for the change, Carpio says.
“There was a clear agreement with this bill, 1187, that it was going away – that they could accomplish what they wanted to do by making it more simplified,” she says. “There was a clear agreement [that] there was supposed to be actions taken. I don’t know what happened in the end.”
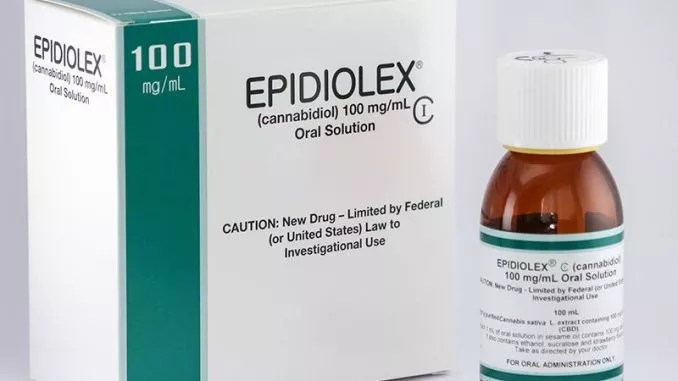
Epidiolex is made from plant-derived cannabidiol, or CBD.
YouTube
While there was talk of a new bill during the meeting, there was also talk of amending the bill to provide protections for the hemp and cannabis industries, Singer says.
“I said that either we’re going to submit a new bill request and see if leadership would approve that, or our nonpartisan bill drafter would write an amendment that comported with the title, [Food And Drug Administration Cannabidiol Drug Use],” he explains. “And [the bill drafter] wrote an amendment that comported with the title.”
The amendment to the bill that was successfully added in committee states that “the General Assembly does not intend for this legislation to be construed so as to prohibit, preclude, or otherwise affect previously authorized activities concerning products derived from marijuana, industrial hemp, or other lawful sources which contain cannabinoids but which are not a prescription medicine approved by the United States Food and Drug Administration.”
“The big fix that we couldn’t do, what I wanted, was to ensure that any FDA-approved drug could be dispensed by a pharmacist regardless of whether it was marijuana or Schedule I, II or whatever,” Singer says. But he couldn’t get it through. “I would much rather have the cleaner language, but at the end of the day I just want to make sure we’re not excluding anyone.”
Singer adds that he received “strong verbal commitment” from stakeholders to go back to the drawing board if there’s unintentional consequences that end up prohibiting the sale of hemp products. “We’ve always got to be really careful to avoid these unintended consequences,” he says. “I hope we threaded the needle on this one.”
Carpio is still worried, however, and felt duped when the bill passed unanimously in the Senate.
“GW Pharmaceuticals isn’t the devil or anything. They spent a lot of money to do what they’re doing, and we respect how they’re trying to take that particular path,” she says. “We’re not against it. We just wanted to hold off for one year. You can access a 10,000 milligram [CBD] bottle here from a state-approved producer.”